The Future of Facial Rejuvenation: Biologic Facelifts, PDRN, and Stem Cell Integration
December 23, 2025
Thanks to leaps in cellular biology, biomaterials, and imaging, facial rejuvenation is moving beyond “cut and tighten” toward true tissue repair. Surgical facelifts still lead the pack for significant laxity and gravitational descent, but a new category—biologic facelifts—leans on growth factors, polynucleotides, exosomes, extracellular matrix (ECM) scaffolds, and biostimulatory injectables to rebuild skin from the inside out. In this piece, we break down the science, protocols, and real-world considerations behind biologic rejuvenation, with a close look at PDRN and stem cell–informed approaches.
The Evolving Paradigm: From Excisional Lifting to Biologic Regeneration
Mechanistic Contrast: Structural Repositioning vs. Cellular Remodeling and Tissue Regeneration
- Surgical lifting repositions descended tissues and removes redundancy—primarily addressing the mechanical drop of the SMAS and skin.
- Biologic treatments stimulate the skin’s own repair programs: fibroblast activation, angiogenesis, ECM remodeling, and immune recalibration that quiet inflammation and improve tissue quality. The goal? Rebuild dermal collagen and elastin, improve blood flow, and normalize the extracellular environment.
In practice, these strategies often meet in the middle. Many patients do best with a hybrid plan—structural lifting paired with biologic therapies that enhance skin quality and extend the life of the results.
Patient Stratification and Indications: Chronologic vs. Biologic Aging, Skin Phenotypes, and Risk Profiles
Choosing the right treatment depends on the interplay of:
- Laxity and descent: Tools like the Merz Facial Laxity Scale help decide whether energy devices/biostimulators are enough—or if surgical elevation is the better bet.
- Skin biology: Dermal thickness, TEWL, elastosis, dyschromia, and vascularity (assessed with high-frequency ultrasound, VISIA/Antera imaging, and cutometry) signal when deeper biologic remodeling is needed.
- Phenotype and risk: Fitzpatrick type, photoaging grade (e.g., Glogau), smoking, glycemic status, autoimmune history, and scarring tendency influence modality selection and complication risk.
Regulatory and Ethical Considerations: Minimally Manipulated Tissue, Off-Label Biologics, and Informed Consent
- In many regions, exosomes, expanded stem cells, and some polynucleotides are still investigational for aesthetic injection. “Minimal manipulation” and “homologous use” rules limit how human cells, tissues, and cellular products can be used outside trials.
- Informed consent should be crystal clear about off-label use, the uncertainty around long-term outcomes, and alternatives. Sourcing transparency (autologous vs. allogeneic) and sterility/quality documentation aren’t optional—they’re essential.

Outcome Endpoints: Objective Imaging, Biomechanical Metrics, and Patient-Reported Measures (FACE-Q)
- Objective measures: high-frequency ultrasound for dermal thickness; cutometry for elasticity (R2, R5); TEWL for barrier function; Antera/VISIA for texture, pigmentation, and redness.
- Patient-reported outcomes: FACE-Q modules (Satisfaction with Facial Appearance, Skin, Psychological Well-Being) capture perceived value and help refine protocols. After all, what’s the point if patients don’t feel the difference?
Biologic Facelifts: Concepts, Modalities, and Protocol Architecture
Defining the Biologic Facelift: Growth Factors, Exosomes, ECM Scaffolds, and Biostimulatory Agents
Think of a biologic facelift as a layered, protocol-driven plan that coaxes regeneration rather than just filling or lifting. Core elements may include:
- Biostimulators (e.g., poly-L-lactic acid, hyperdilute CaHA) for neocollagenesis and neoelastogenesis.
- Polynucleotides/PDRN to modulate inflammation, accelerate repair, and support angiogenesis.
- Growth factor–rich preparations and investigational exosomes to influence cell signaling.
- ECM scaffolds (collagen-based, hyaluronic acid matrices) to guide remodeling with structural cues.
- Synergistic devices (microneedling, RF, ultrasound) to recruit wound-healing pathways and improve delivery.
Mechanisms of Action: Fibroblast Activation, Angiogenesis, ECM Remodeling, and Immunomodulation
- Fibroblast activation: Mechanical and biochemical cues elevate collagen I/III and elastin synthesis, improving tensile strength.
- Angiogenesis: VEGF and related pathways enhance perfusion and nutrient delivery—key for sustained remodeling.
- ECM remodeling: Controlled microinjury and biostimulation balance MMP activity and encourage organized collagen deposition.
- Immunomodulation: Lower pro-inflammatory cytokines (e.g., TNF-α) and stronger regulatory signaling steer healing toward regeneration rather than fibrosis.
Procedure Design: Layered Delivery, Device Synergy, and Aftercare
A common architecture looks like this:
- Priming: Strict UV protection, barrier repair, pause pro-inflammatory topicals. Consider energy-based tightening (e.g., MFU-V ultrasound) to “reset” tissue tension.
- Deep biostimulation: Hyperdilute CaHA in the subdermal plane to upregulate collagen/elastin; PLLA in deep subcutaneous planes for volumetric biostimulation when needed.
- Dermal remodeling: Intradermal polynucleotides/PDRN micro-depots plus microneedling RF to wake up fibroblasts and enhance delivery.
- Contour refinement: Low-G′ HA for superficial lines or MLF (myomodulatory filler) techniques as appropriate.
- Maintenance: Quarterly to biannual biologic “boosters,” with periodic energy-device touch-ups.
Aftercare focuses on photoprotection, gentle barrier support, antiviral prophylaxis when needling around the mouth/nose, and avoiding heavy manipulation or heat for 48–72 hours. Simple, sensible, effective.
Risk Management: Infection Control, Biofilm and Granuloma Mitigation, and Complication Pathways
- Asepsis is non-negotiable. If the skin barrier is compromised, rethink device-then-injectable sequencing—stagger when in doubt.
- Biofilm risk rises when fillers and biologics share the same session and plane. Separate sessions or planes; consider ultrasound guidance in higher-risk areas.
- Nodule/granuloma prevention: Choose the right product and depth; keep volumes conservative; avoid superficial PLLA/CaHA. Manage early with hyaluronidase (HA fillers), intralesional steroids/5-FU for biostimulator nodules; use culture-guided antibiotics if biofilm is suspected.
- Vascular safety is paramount—respect safe zones, use cannulas when appropriate, aspirate judiciously, and keep hyaluronidase on hand.
PDRN (Polydeoxyribonucleotide) in Aesthetic Medicine
Pharmacology and Mechanisms: A2A Receptor Signaling, Nucleotide Salvage, Anti-Inflammatory Effects
PDRN consists of DNA fragments (often salmon-derived) typically ranging from 50–1500 kDa. Two key actions drive its effects:
- Adenosine A2A receptor activation: Switches on pro-repair pathways, boosting angiogenesis (e.g., VEGF upregulation) and dialing down pro-inflammatory cytokines (TNF-α, IL-6).
- Nucleotide salvage: Supplies building blocks for DNA/RNA synthesis—fuel for fibroblast proliferation and DNA repair.
Clinically, that means better dermal matrix quality, quicker wound resolution, and more even pigmentation in dysregulated skin. Note: In East Asia, polynucleotide (PN) products with similar biology are widely used; formulations and regulatory status differ by country.
Formulations and Delivery: Mesotherapy, Intradermal Micro-Depots, Cannula Techniques, HA Combinations
Common delivery strategies include:
- Mesotherapy/micro-depots: 0.01–0.05 mL intradermal blebs spaced 0.5–1.0 cm apart, roughly ~1–2 mL for the full face per session.
- Cannula “fanning” in the deep dermis for even spread with less bruising—great for high-visibility patients.
- Pairing with low-crosslinked HA (“skin boosters”) to combine hydration with biologic signaling.
- Synergy with microneedling or fractional RF can improve penetration—but time it wisely to reduce infection risk and product loss.
Clinical Evidence: Skin Elasticity, TEWL, Photoaging Markers, Scar Remodeling, and Pigment Modulation
Smaller randomized and prospective studies report:
- Increased dermal thickness and improved elasticity (cutometry and ultrasound).
- Reduced TEWL and better barrier metrics within weeks.
- Improvements in fine lines and texture, especially around the eyes and cheeks.
- Adjunctive benefits for acne scars, striae, and post-inflammatory dyschromia when combined with microneedling or laser.
The evidence base is growing, but variations in formulations (PDRN vs. PN), dosing, and endpoints highlight the need for standardized trials. Science takes time—this is moving in the right direction.
Safety, Dosing, and Protocols: Contraindications, Treatment Intervals, and Combination Strategies
- Typical course: 3–4 sessions every 2–4 weeks, then maintenance every 3–6 months based on response and lifestyle.
- Adverse effects: Transient erythema, edema, pinpoint bleeding, bruising; rare hypersensitivity. Because of marine origins, some clinicians use caution in fish-allergic patients (immunogenicity appears low).
- Contraindications: Active skin infection, poorly controlled autoimmune disease, pregnancy/lactation, and known hypersensitivity to components.
- Combinations: Commonly paired with energy devices, HA boosters, or biostimulators—sequence to limit biofilm risk and avoid stacking inflammation (e.g., separate RF and injectables by 1–2 weeks).
Stem Cell Integration: Autologous and Allogeneic Strategies
Cell Sources and Status: ADSC/SVF, Bone Marrow, Perinatal Derivatives; Minimal Manipulation vs. Expansion
- Autologous adipose sources: Stromal vascular fraction (SVF) contains MSC-like cells, pericytes, and endothelial progenitors. Mechanical methods (e.g., nanofat) may meet “minimal manipulation” standards in some regions; enzymatic isolation and ex vivo expansion typically require regulatory approvals.
- Bone marrow–derived MSCs: Powerful but more invasive to harvest; expansion generally falls under advanced therapy regulations.
- Perinatal derivatives (umbilical cord/Wharton’s jelly): Allogeneic with variable regulatory status—promising secretome, but they demand rigorous quality controls.
Delivery Paradigms: Cell-Assisted Lipotransfer, Conditioned Media, Exosomes, and ECM Scaffolds
- Cell-Assisted Lipotransfer (CAL): Enriching fat grafts with SVF to improve survival and quality—especially useful for midface restoration, temples, and scarred regions.
- Conditioned media and exosomes: Cell-free biologics delivering signaling molecules; attractive for safety and standardization, though injectable exosomes are largely unapproved at present.
- ECM scaffolds: Collagen or HA matrices can host cells or cell-free biologics, prolonging residence time and guiding tissue organization.
Manufacturing and Quality: GMP Compliance, Release Criteria, Potency Assays, and Standardization
- GMP facilities should manage donor screening; sterility (USP <71>); endotoxin and mycoplasma testing; and identity/viability checks.
- Release criteria: For MSCs—CD73/CD90/CD105 positivity with hematopoietic marker negativity and defined viability thresholds; for exosomes—particle size/count (NTA), morphology (TEM), markers (CD63/CD81/TSG101).
- Potency assays: Functional tests (fibroblast proliferation/migration, angiogenesis tube formation, cytokine modulation) ensure clinical relevance.
- Standardization challenges: Donor age, metabolic health, and processing methods can dramatically alter the secretome’s potency and safety. Consistency is half the battle.
Long-Term Safety: Tumorigenicity, Ectopic Differentiation, Immunogenicity, and Donor Variability
- Properly characterized MSCs show low tumorigenicity risk, but long-term follow-up remains wise.
- Ectopic differentiation and calcification are theoretical risks when delivery is off-target or poorly controlled.
- Allogeneic therapies may be immune-evasive—but not invisible; sensitization can occur.
- Donor variability is a major source of outcome differences—another reason cell-free, standardized products may ultimately win out once regulations catch up.
Clinical Pathways, Implementation, and Future Directions
Assessment Algorithms: Laxity Staging, Dermal Quality, Photodamage Grading, and Contraindications
A reproducible intake pathway might include:
- Laxity and descent: Merz scale (or similar) plus photographic and ultrasound mapping.
- Skin quality: TEWL, cutometry, VISIA/Antera for texture/pore/vascular/pigment mapping; 20–25 MHz ultrasound for dermal thickness.
- Photodamage: Glogau type; assess melanosis and vascular contributions to dyschromia.
- Contraindications: Active dermatoses, keloid tendency (relevant for needling/biostimulators), pregnancy, recent isotretinoin (device-dependent), coagulopathy, and active autoimmune disease.
Treatment Sequencing: Biostimulators, Energy-Based Devices, Neuromodulators, and Maintenance
A smart sequence to minimize interference and risk:
- Foundation: Neuromodulators for dynamic lines; normalize skincare; enforce sun protection.
- Energy-based tightening: MFU-V or RF for lifting; fractional modalities for texture when indicated.
- Biostimulators: Hyperdilute CaHA or PLLA to build scaffold and spark neocollagenesis.
- Dermal optimization: PDRN/PN intradermal series; selective HA boosters for hydration and glow.
- Refinement: HA fillers for contour, lasers for pigment/vessels, and targeted scar work.
- Maintenance: Quarterly biologic boosts and annual device refreshers guided by objective metrics.
Example: A 52-year-old outdoor athlete with mild jowl descent, perioral lines, and photoaging has MFU-V first, then hyperdilute CaHA to the lower face, followed by three PDRN sessions three weeks apart. Low-G′ HA treats lip lines after the second PDRN. Results? Increased dermal thickness on ultrasound and higher FACE-Q satisfaction at 12 weeks.
Outcomes and Study Design: Imaging (VISIA/Antera), Ultrasound, Cutometry, and Real-World Evidence
- Standardize pre/post imaging: lighting, hydration, positioning—control the variables you can.
- Primary endpoints: Dermal thickness change on ultrasound; elasticity (R2) on cutometry; TEWL reduction.
- Secondary endpoints: Texture and redness improvements on Antera/VISIA; FACE-Q domains; downtime and adverse event rates.
- Real-world data: Prospective registries with 6–12 month follow-up help fill the gap while randomized trials mature.
Operational Considerations: Economics, Pricing Models, Patient Education, and Informed Consent
- Packaging: Offer phased programs (e.g., a 12-week biologic facelift) rather than one-offs; include imaging to demonstrate progress and value.
- Inventory and cold chain: Many biologics and injectables need validated storage—track lot numbers and expiry religiously.
- Training and SOPs: Cannula vs. needle protocols, device settings, emergency algorithms, ultrasound guidance competencies—make them routine.
- Education: Explain the “how” and the timeline—biologic remodeling is gradual. Set expectations around maintenance and potential combined surgical/biologic plans.
- Documentation: Detailed consents covering off-label status, product sourcing, potential complications, and alternatives keep everyone aligned.
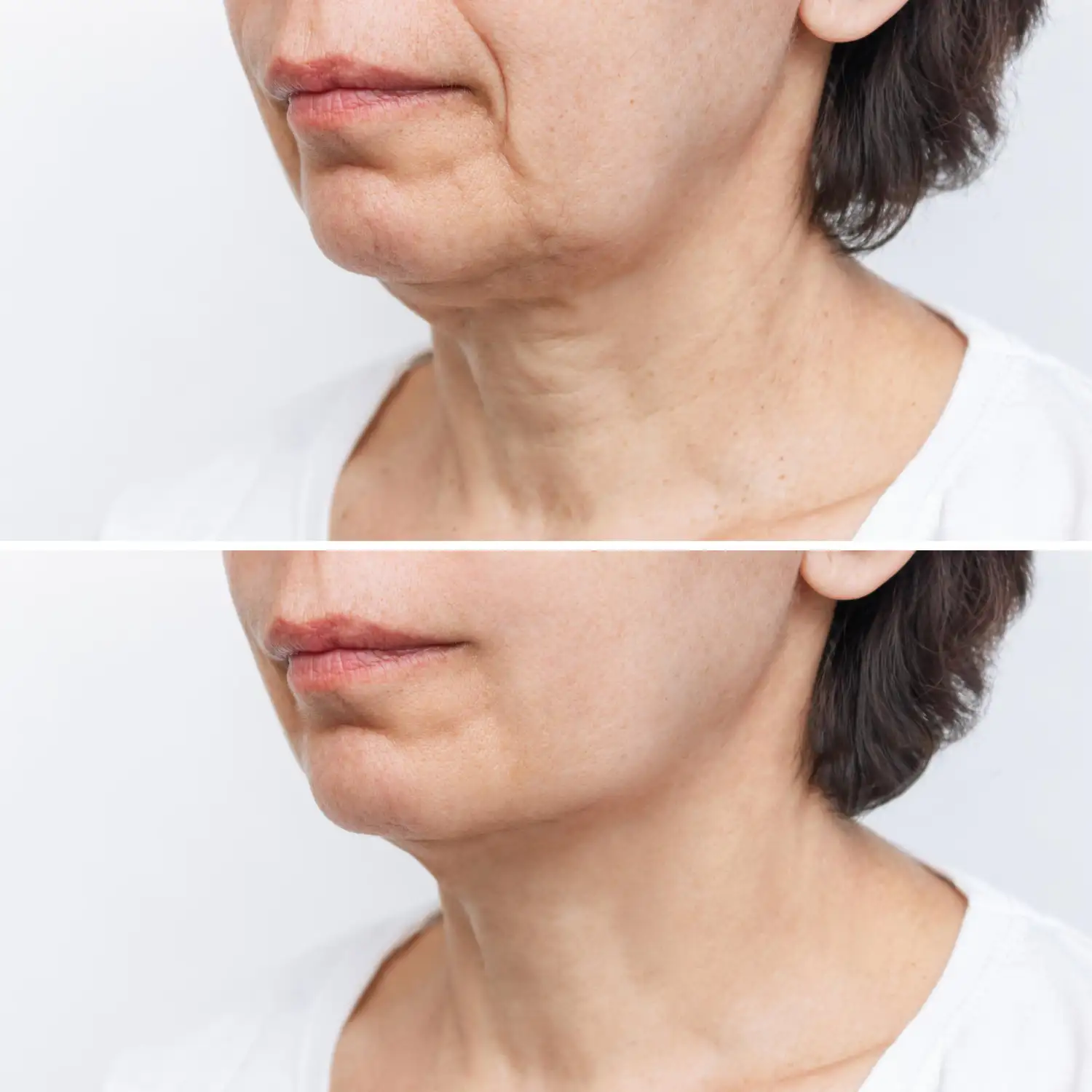
Conclusion
Facial rejuvenation is shifting from simply pulling tissues tighter to rebuilding them healthier. Biologic facelifts—thoughtful combinations of biostimulatory injectables, polynucleotides like PDRN, growth factor–based strategies, and well-sequenced energy devices—offer a credible path to regenerative results with minimal downtime. Stem cell–based integrations, from cell-assisted fat transfer to cell-free exosome approaches, are promising but require rigorous manufacturing, standardization, and regulatory oversight.
The future belongs to protocols that are as measurable as they are artful. Clinics that embrace objective imaging, biomechanical assessments, and validated patient-reported outcomes won’t just deliver better results—they’ll help build the evidence base this field needs. As regulations evolve, the marriage of safe, standardized biologics with precision delivery and robust analytics will make “regeneration over resection” a practical, scalable reality for the right patients. Ready to rebuild, not just lift?

Schedule Your Appointment with Dr. Mourad
If you are considering facial plastic surgery and want results that enhance your natural beauty without looking overdone, schedule a consultation with Dr. Moustafa Mourad today. You will receive personal, expert guidance at every step—from your first visit to your final result.
From Our Blog

February 6, 2026 | Dr. Moustafa Mourad | Uncategorized
Facelift and Neck Lift: Why They’re Often Done Together
A well-done lower facelift can sharpen the jawline, soften jowls, and restore midface support. A precise neck lift can refine the cervicomental angle and correct banding or fullness under the chin.
READ THE ARTICLE
February 5, 2026 | Dr. Moustafa Mourad | Uncategorized
Mini Facelift vs. Full Facelift: How to Choose the Right Procedure for You
A sharp jawline isn’t just an aesthetic ideal—it’s the visible result of how skin, fat, fascia, ligaments, and bone work together. With time, that system loses tension and volume.
READ THE ARTICLE
February 3, 2026 | Dr. Moustafa Mourad | Uncategorized
Facelift Recovery Day by Day: Realistic Timelines and What to Expect
Choosing a facelift is both a beauty decision and a promise you make to your future self. The surgeon’s skill shapes the result—but your recovery determines when you get to see it.
READ THE ARTICLE
February 2, 2026 | Dr. Moustafa Mourad | Uncategorized
What’s the Best Age for a Facelift? Follow the Clues in Your Skin and Anatomy
Picking the “right” age for a facelift isn’t about the number on your birthday cake—it’s about what your tissues can still do. Skin elasticity, the strength of your SMAS (the facial support layer)
READ THE ARTICLE