How AI Is Changing Facelift Planning: From 3D Simulation to Predictive Healing Models
December 30, 2025
Artificial intelligence is reshaping aesthetic surgery far beyond photo filters and those glossy before–after galleries. In facelift planning especially, AI now covers the whole journey—from high-fidelity 3D capture and biomechanical simulation to models that predict when swelling will settle and how scars will mature. The payoff? A more informed consultation, more precise execution in the OR, and more proactive follow-up. In the pages ahead, we’ll unpack the technical foundations, the day-to-day clinical workflow, and the governance issues that matter as AI moves from novelty to standard of care.
From Imaging to Simulation: The New Technical Foundation of Facelift Planning
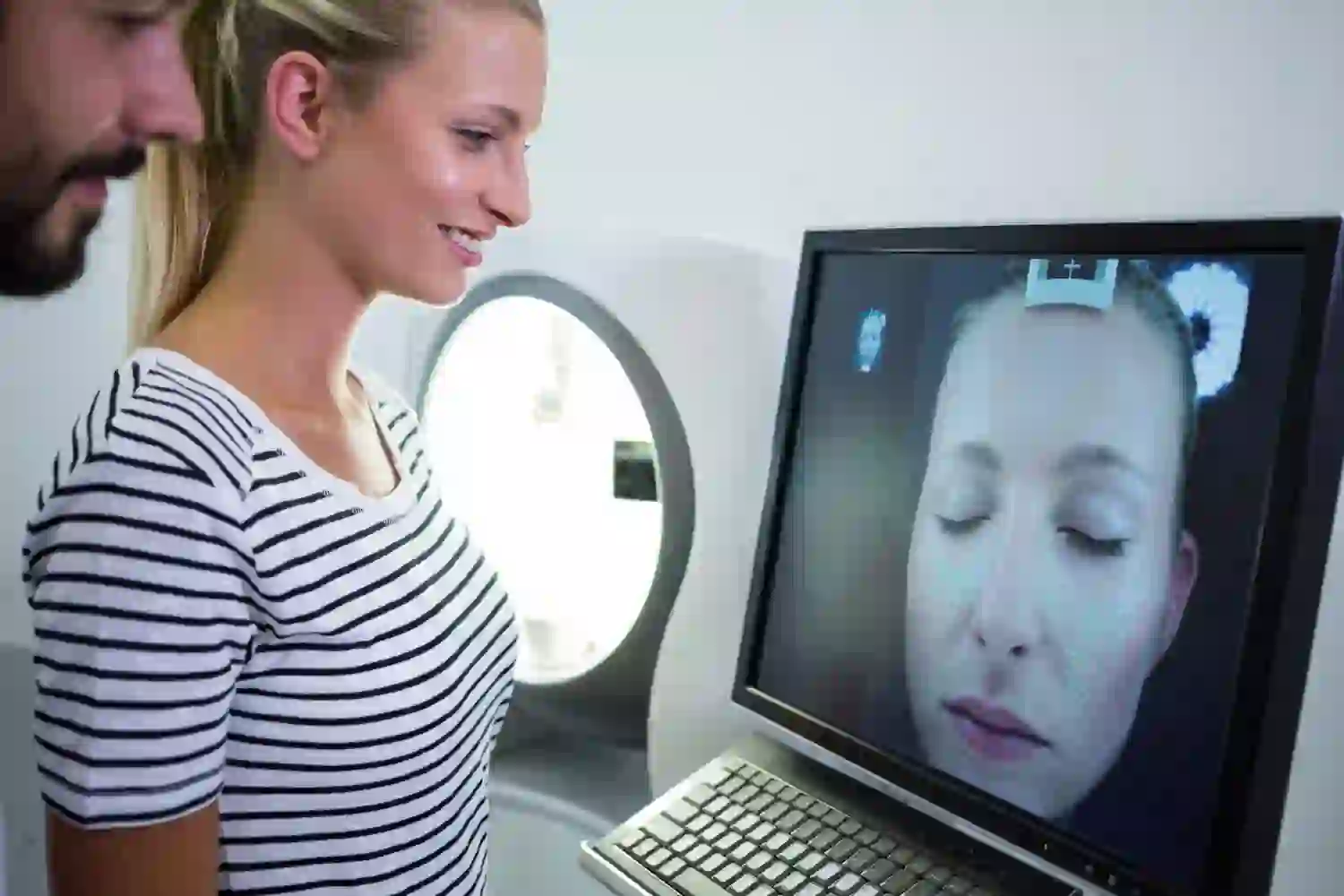
Acquisition and Registration Pipelines: 3D Photogrammetry, Structured Light, CBCT/MRI, and Landmarking
Modern planning starts with geometry you can trust. Two non-ionizing workhorses lead the way:
- 3D photogrammetry reconstructs a dense surface from multiple calibrated photos. It’s cost-effective and captures true color/texture.
- Structured light scanners project patterns onto the face and infer depth from how those patterns deform—often with higher accuracy and less sensitivity to lighting.
Need deeper anatomy? Cone-beam CT (CBCT) gives reliable bony landmarks at relatively low dose, while MRI maps soft tissue planes, parotid anatomy, and deep fat compartments—handy for complex or revision cases. Toolchains then register these modalities into a common coordinate system via:
- Rigid alignment (Procrustes, iterative closest point) to align surfaces.
- Nonrigid registration to reconcile expressions or posture shifts using free-form deformations.
- Landmarking of perioral, periorbital, mandibular, and zygomatic points—first detected automatically by CNN-based landmarkers, then refined manually for clinical fidelity.
Well-validated pipelines can hit millimeter-level surface error under controlled conditions. That matters when a 2–3 mm change at the jowl or marionette line is clinically perceptible. Small differences, big impact.
Generative and Morphable Models for Preoperative Visualization: 3DMMs, NeRFs, and Diffusion/GAN Approaches
Once the face is registered, AI-driven generative models unlock patient-specific visualization:
- 3D Morphable Models (3DMMs) compress facial shape and albedo into low-dimensional parameters learned from large datasets. Clinicians can adjust “shape” coefficients to mimic planned maneuvers (e.g., superolateral SMAS vectoring) while preserving the patient’s identity and proportions.
- Neural Radiance Fields (NeRFs) learn a volumetric representation from multi-view images, producing photorealistic renderings under new viewpoints and lighting—great for setting expectations from every angle.
- Diffusion and GAN-based methods can synthesize textures (say, skin quality shifts) and harmonize lighting. With the right constraints, the “after” portrait matches 3D geometry—no freehand fakery.
The best systems combine morphable shape models with volumetric or neural rendering so you get clinically plausible geometry and lifelike visuals—without the “uncanny valley” of 2D edits. Because who wants a beautiful rendering that isn’t anatomically believable?
Biomechanical Soft-Tissue Modeling: FEM of Skin–SMAS–Ligament Systems and Constitutive Laws
Pretty pictures aren’t enough when skin, SMAS, and retaining ligaments respond nonlinearly to surgical forces. Finite element models (FEM) estimate how these layers deform under suture tension, undermining, release, and vectoring. Typical elements include:
- Layered structures: epidermis/dermis (anisotropic, aligned with Langer’s lines), subcutaneous fat (near-incompressible), SMAS and platysma (fiber-reinforced), and retaining ligaments (zygomatic, mandibular, masseteric).
- Boundary conditions: fixation to bony landmarks (zygoma, mandible) and deep fascia; incision edges; suture anchor points on the SMAS.
- Constitutive behavior: hyperelastic (neo-Hookean, Ogden) for soft tissue; viscoelasticity via Prony series to capture creep and relaxation; directional stiffness for collagen-heavy layers.
In practice, surgeons can run “what if” scenarios—e.g., a 20–30° superolateral SMAS vector with a target jowl displacement—then preview predicted skin redraping, nasolabial fold softening, and tension along incision lines. The goal isn’t biological perfection; it’s a patient-specific, physics-informed forecast that beats intuition alone. Helpful? Absolutely.
Validation Protocols: Surface Distance Metrics, Anthropometric Fidelity, and Intraoperative Ground Truth
Credibility demands validation:
Statistical tools like Bland–Altman plots test agreement; intra- and inter-rater reliability for landmarking ensures robustness. Clinical systems should report accuracy transparently (e.g., “1.3 ± 0.6 mm vertex-wise error over the lower face”). No smoke and mirrors—just numbers.
- Surface metrics: mean absolute surface distance, root-mean-square error, and Hausdorff distance between simulated and actual postoperative scans.
- Anthropometric checks: changes in gonial angle, lower facial third measurements, marionette fold depth, and submental plane thickness.
- Intraoperative ground truth: optical scanning of lifted flaps before closure; perfusion maps; standardized photographs for landmark verification.
Statistical tools like Bland–Altman plots test agreement; intra- and inter-rater reliability for landmarking ensures robustness. Clinical systems should report accuracy transparently (e.g., “1.3 ± 0.6 mm vertex-wise error over the lower face”). No smoke and mirrors—just numbers.
Predictive Healing and Outcome Modeling: From Edema Trajectories to Scar Quality
Multimodal Feature Sets: Demographics, Comorbidities, Intraoperative Variables, Perfusion and Vascularity Signals
Healing models work best when fed diverse signals:
Put together, these streams give richer predictions than any single variable could. Because the body doesn’t heal in a vacuum.
- Patient factors: age, sex, BMI, Fitzpatrick skin type, smoking, diabetes, autoimmune conditions, medications (anticoagulants, corticosteroids).
- Intraoperative factors: duration, extent of undermining, drain placement, anesthesia type, infiltrate composition (e.g., lidocaine/epinephrine), suture materials, and tension estimates.
- Tissue signals: indocyanine green (ICG) fluorescence angiography, laser speckle contrast imaging, capillary refill times, thermal imaging, and intraoperative bleeding patterns.
- Early postoperative signals: smartphone-based swelling indices, ecchymosis color metrics, and wound-edge perfusion trends.
Put together, these streams give richer predictions than any single variable could. Because the body doesn’t heal in a vacuum.
Temporal and Digital Twin Methods: Survival Analysis, Sequence Models, and Mechanobiological Coupling
Time is everything. Rather than predicting a single endpoint, modern models forecast trajectories:
Digital twin frameworks bring it all together: initialize a patient-specific model pre-op and update it post-op as evidence comes in—tightening prediction intervals as you go. A living model of healing, not a one-off guess.
- Survival analysis (Cox models, accelerated failure time) for time-to-edema-baseline or time-to-bruising-clearance.
- Sequence models (gradient-boosted time series, LSTM/transformers) that ingest daily photos, vitals, and symptom check-ins—updating predictions as new data arrives.
- Mechanobiological coupling links mechanical tension and perfusion to biology (inflammation, collagen deposition, scar remodeling) for more causal plausibility. Example: high incision-line tension plus low ICG perfusion may flag risk for delayed healing or widened scars, prompting earlier interventions (taping, silicone therapy, tension-reduction strategies).
Digital twin frameworks bring it all together: initialize a patient-specific model pre-op and update it post-op as evidence comes in—tightening prediction intervals as you go. A living model of healing, not a one-off guess.
Clinical Endpoints and Scales: Edema Resolution, Ecchymosis, Scar Maturation, Nerve Function; FACE-Q and GAIS
Predictions should map to endpoints that matter:
- Edema and ecchymosis: volumetric swelling reduction back to baseline (via 3D scans) and colorimetric bruising measures.
- Scar quality: patient and observer scales (e.g., Patient and Observer Scar Assessment Scale), plus texture/color metrics; many practices add standardized photo grading.
- Nerve function: branch-specific motor exams with standardized maneuvers and strength grading.
- Patient-reported outcomes: FACE-Q domains (satisfaction with face, psychological well-being) and the Global Aesthetic Improvement Scale (GAIS) to capture perceived change.
Uncertainty Quantification and Model Calibration: Bayesian Approaches, Conformal Prediction, and Error Bounds
Predictions without uncertainty can mislead. Best practice includes:
Spell out uncertainty plainly. Patients and surgeons both make better choices with honest ranges, not false precision.
- Bayesian models or ensembles to provide credible intervals (e.g., “edema to baseline in 18–24 days”).
- Conformal prediction for distribution-free, finite-sample error guarantees—especially useful in heterogeneous populations.
- Calibration checks (reliability diagrams, expected calibration error) and subgroup analyses to ensure risk scores match observed frequencies across skin types and ages.
Spell out uncertainty plainly. Patients and surgeons both make better choices with honest ranges, not false precision.
Clinical Workflow Integration: Decision Support from Consultation to Follow-Up
Preoperative Consultation and Shared Decision-Making: Scenario Simulation and Expectation Alignment
During consultation, surgeons can:
- Demonstrate multiple vectoring strategies (e.g., superolateral SMAS tightening vs. deep plane release) and show how they differently affect jowling and neck contour.
- Overlay predicted scar trajectories and camouflage strategies.
- Use outcome distributions to frame risk: “Patients with similar characteristics had X% probability of residual submental fullness at 3 months.”
Intraoperative Guidance: AR Overlays, Suture Vector Optimization, and Perfusion Monitoring Integration
In the OR, AI augments judgment—it doesn’t replace it:
- AR overlays anchored to facial landmarks can display planned incision lines, target vectors, and areas of predicted high tension. Heads-up displays or tablet-based AR keep attention where it belongs.
- Suture vector optimization tools translate planned displacements into suggested vector ranges and anchor points, based on precomputed biomechanical maps.
- Perfusion integration: real-time ICG angiography or laser speckle imaging can alert when perfusion drops below thresholds tied to delayed healing—guiding flap handling and closure strategy.
It’s guidance at the point of action, especially valuable in complex revisions or asymmetry. Small nudges, fewer surprises.
Postoperative Monitoring: Smartphone Capture, Computer Vision for Wound/Swelling, and Telehealth Protocols
Standardized at-home capture—same lighting, distance, head position—feeds computer vision models that:
- Quantify swelling volumes and color shifts as bruising resolves.
- Monitor incision lines for dehiscence risk, erythema trends, or drainage.
- Flag outliers for telehealth check-ins or earlier in-person assessment.
Automated reminders and adherence tracking keep data quality high while cutting low-value visits. Your team focuses on what really needs attention.
Interoperability and Infrastructure: DICOM/FHIR Integration, PACS Connectivity, Edge vs Cloud Compute
To avoid AI becoming a silo, make it seamless:
- Imaging: DICOM-based ingestion for CBCT/MRI; 3D photo workflows that store meshes with metadata.
- EHR: FHIR APIs to pull demographics, comorbidities, and intraoperative notes—and push structured predictions and tasks back to the chart.
- PACS/VNA connectivity for archival and enterprise access.
- Compute strategy: edge processing for low latency and privacy (e.g., OR AR overlays), cloud for heavier training and cohort analytics. A hybrid model with containerized deployments and GPU scheduling balances performance and cost.

Safety, Ethics, and Regulatory Compliance for AI in Aesthetic Surgery
Data Governance and Privacy: HIPAA/GDPR Compliance, Consent Models, De-Identification, and Federated Learning
Facial data is inherently identifiable. Programs should enforce:
- Explicit informed consent specifying AI uses, retention periods, and opt-out options.
- Robust de-identification where possible (metadata scrubbing, face masking for non-facial images), plus encryption at rest and in transit.
- HIPAA-compliant Business Associate Agreements and GDPR lawful bases (consent or legitimate interest), with full support for data subject rights.
- Federated learning or on-prem training to limit centralization of raw data—layer in differential privacy where feasible.
Bias and Fairness: Performance Across Fitzpatrick Skin Types, Ages, Sexes, and Diverse Anatomies
Biased models risk unequal care. Mitigate by:
- Building diverse training sets across Fitzpatrick I–VI, craniofacial variation, age ranges, and sex.
- Reporting subgroup performance with parity targets (e.g., comparable edema prediction error across skin types).
- Using domain-specific augmentations (illumination, color) and device normalization to reduce capture bias.
- Monitoring for drift as patient populations and imaging devices evolve.
Regulatory Pathways and Standards: FDA SaMD (510(k)/De Novo), GMLP, ISO 14971 Risk, IEC 62304 Software Lifecycle
Many of these tools qualify as Software as a Medical Device (SaMD). Key points:
- U.S. FDA: 510(k) if a predicate exists; De Novo otherwise. Predetermined Change Control Plans (PCCPs) can allow regulated ML updates.
- Good Machine Learning Practice (GMLP): strong data management, train/validation separation, clinically meaningful endpoints, real-world performance monitoring.
- ISO 14971 for risk management; IEC 62304 for software lifecycle; IEC 62366 for usability; plus recognized cybersecurity frameworks.
- Postmarket surveillance to catch adverse trends, with corrective actions and documented change control.
Human Oversight and Explainability: Surgeon-In-The-Loop, Audit Trails, and Transparent Model Behavior
AI should stay in an assistant role:
- Surgeon-in-the-loop workflows with override capability and rationale capture.
- Audit trails of inputs, model versions, and outputs for traceability and quality improvement.
- Explainability tailored to modality: saliency maps for imaging; feature attributions (e.g., SHAP) for tabular risk models; intuitive visualizations of uncertainty.
Business Impact, Implementation Roadmap, and Future Horizons
Economic Value Proposition: Case Acceptance, Planning Efficiency, Reoperation Reduction, and KPI Frameworks
Done right, an AI platform pays its way:
- Higher case acceptance via personalized, realistic visualization.
- Planning efficiency—minutes saved per case add up fast; standardized templates reduce variability.
- Fewer revisions by de-risking asymmetries and tension hotspots.
- KPI dashboards tracking: conversion rates, planning time, revision/complication rates, time-to-edema-baseline, FACE-Q improvements, provider workload.
Change Management and Training: Clinical Adoption, Credentialing, and Cross-Functional Governance
Adoption is about people and process:
- Identify clinical champions; run simulation labs and proctored cases.
- Credential users with competency checks on capture protocols, interpreting uncertainty, and AR use.
- Stand up governance across surgery, nursing, IT, legal, and quality; define escalation paths and downtime procedures.
Build vs Buy Decisions: Vendor Due Diligence, SLAs, Validation Studies, and MLOps for Continuous Improvement
Make smart strategic choices:
Today’s decision sets tomorrow’s speed and safety.
- Vet vendors for clinical validation, data governance, security certifications, and reference sites.
- Lock in SLAs covering uptime, support, model update cadence, and issue resolution.
- Clarify data rights and IP for patient data and derived models.
- Ensure MLOps capabilities—versioning, monitoring, drift detection, A/B testing—whether in-house or via a partner.
Today’s decision sets tomorrow’s speed and safety.
Future Directions: Real-Time Intraoperative Biomechanics, Robotic Assistance, Multispectral Imaging, and Closed-Loop Systems
The next wave is already on the horizon:
- Real-time FEM approximations on GPUs to update deformation predictions as tissues are manipulated.
- Robotic assistance for steady, quantifiable retraction or suture placement under AI guidance—with force and perfusion feedback.
- Multispectral and hyperspectral imaging to map oxygenation and hemoglobin without dyes.
- Closed-loop systems that adapt intraoperative strategy based on live perfusion, tension, and predicted healing—always with human oversight.
What happens when guidance becomes truly interactive? We’re about to find out.

Conclusion
AI is moving facelift planning from educated guesswork to measurable, patient-specific decision support. High-fidelity capture and biomechanical simulation enable realistic scenario planning. Predictive models turn perioperative signals into useful forecasts of edema, scarring, and patient-reported satisfaction. Integrated into consults, the OR, and follow-up, these tools can elevate outcomes and streamline workflow—so long as we govern them with rigor, fairness, and transparency.
Surgeons who invest now—auditing data quality, validating models against their own outcomes, and training their teams—won’t just improve today’s cases. They’ll help define the standards for aesthetic surgery’s AI-enabled future.

Schedule Your Appointment with Dr. Mourad
If you are considering facial plastic surgery and want results that enhance your natural beauty without looking overdone, schedule a consultation with Dr. Moustafa Mourad today. You will receive personal, expert guidance at every step—from your first visit to your final result.
From Our Blog

February 18, 2026 | Dr. Moustafa Mourad | Uncategorized
How to Choose the Best Facelift Surgeon Near You: A Practical, Evidence‑Based Guide
A great facelift can be life-changing—and it’s one of the most technically demanding procedures in aesthetics. Results depend as much on a surgeon’s judgment,
READ THE ARTICLE
February 16, 2026 | Dr. Moustafa Mourad | Uncategorized
What Is the SMAS and Why It Matters in Facelift Surgery
Facelift surgery has come a long way from simply pulling skin tighter. The best, most natural results today are built on understanding a deeper support layer called the SMAS—the superficial musculoaponeurotic system.
READ THE ARTICLE
February 14, 2026 | Dr. Moustafa Mourad | Uncategorized
What Is the Deep Plane Facelift and Why Surgeons Call It the Gold Standard
Aging in the lower face and neck isn’t just about “extra skin.” It’s about the deeper support system loosening—ligaments stretching, soft tissues dropping, and volume shifting around.
READ THE ARTICLE
February 12, 2026 | Dr. Moustafa Mourad | Uncategorized
How Long Does a Facelift Last? The Truth About Longevity and Maintenance
A facelift is one of the most reliable ways to sharpen the jawline, smooth the midface, and refine the neck. But how long do the results actually last?
READ THE ARTICLE